Psoriasis is a long-term skin disease that causes red itchy scaly patches on the elbows, trunk, scalp, and knees. It is a non-communicable, chronic, disfiguring, and painful disease that has no cure and has a greater negative impact on the quality of life of patients. This illness can occur at any stage but it is most common among people within the age group of 50–69 years. This disease affects around 0.09%–11.4% of the population of the countries. The high prevalence of this skin disease generates a high requirement for psoriasis drugs.
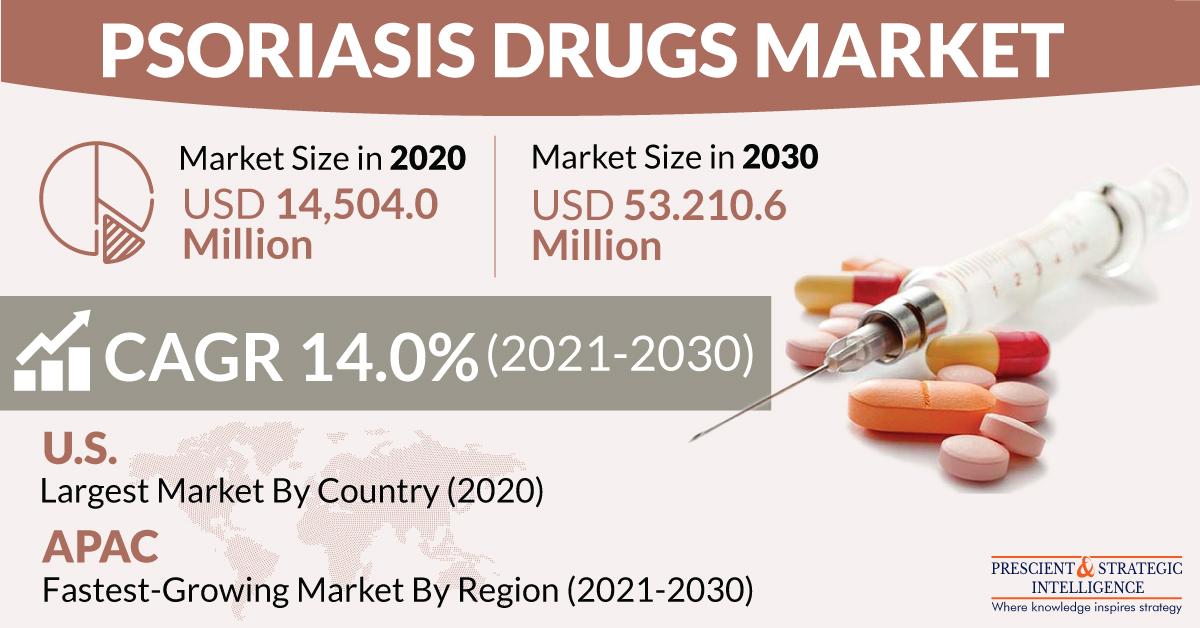
As this disease is highly prevalent in the 50–69 years age group, the increasing elderly population will accelerate the psoriasis drugs market at a CAGR of 14.0% during 2021–2023. The market was valued at $14,504.0 million in 2020 and it is projected to reach $53,210.6 million by 2030. Since the functionality of human skin decreases with age, old people witness slow healing of wounds, increased sensitivity of ultraviolet (UV) radiation, loss of subcutaneous fat, and high susceptibility to skin infections and ailments, such as psoriasis.
In recent years, the surging incidence of psoriasis has led to a soaring number of drug approvals. The rising number of approvals is leading to the expansion of the drug portfolio which in turn is attracting more healthcare professionals and patients. For example, in January 2021, European Commission (EC) approved RINVOQ (upadacitinib - 15 mg) of AbbVie Inc. This psoriasis drug is a selective and reversible Janus kinase (JAK) inhibitor that is administered orally to adults suffering from psoriatic arthritis (PsA).
The rising cases of psoriasis will also result in the largescale production of biologic drugs. Additionally, the rising number of research activities to decode the genetics of psoriasis has led to the development of highly effective and targeted drugs. Thus, the availability of an enhanced treatment to clear psoriasis and decrease the risk of its comorbidities, including inflammatory bowel disease (IBD), PsA, and some cancers, has amplified the usage of biologic drugs among a vast population base.
Additionally, the manufacturers of the medical drug such as Novartis AG, AbbVie Inc., Amgen Inc., Eli Lilly and Company, Bristol-Myers Squibb Company, Merck & Co. Inc., Pfizer Inc., Johnson & Johnson, GlaxoSmithKline plc, Bausch Health Companies Inc., and Mylan N.V. are also developing and offering topical therapies and small-molecule systemic drugs. Drugs manufactured by these companies can be administered through oral, topical, and parenteral routes. For instance, TREMFYA (guselkumab) by Janssen, the pharmaceutical division of Johnson & Johnson, is administered through the parenteral route in adult patients with active PsA.
Download sample pages of this report: https://www.psmarketresearch.com/market-analysis/psoriasis-drugs-market-outlook/report-sample
Geographically, North America consumed the highest quantity of psoriasis drugs in the past, due to the escalating awareness about newly developed drugs and soaring per capita income in the region. Whereas, Asia-Pacific (APAC) is expected to demonstrate the fastest growth in the psoriasis drugs market. This can be ascribed to the mounting healthcare expenditure, mushrooming disposable income, and rising number of product launches in the region. Besides, the booming expenditure on skincare products and the growing presence of leading drug manufacturers in APAC will boost the production of psoriasis drugs in the region.
Thus, the increasing prevalence of psoriasis and the burgeoning elderly population will fuel the need for psoriasis drugs in the foreseeable future.